Market Oriented Policies Agains Sulfur Dioxide
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name Sulfur dioxide | |
| Other names Sulfurous anhydride | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| Beilstein Reference | 3535237 |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.028.359 |
| EC Number |
|
| E number | E220 (preservatives) |
| Gmelin Reference | 1443 |
| KEGG |
|
| MeSH | Sulfur+dioxide |
| PubChem CID |
|
| RTECS number |
|
| UNII |
|
| Un number | 1079, 2037 |
| CompTox Dashboard (EPA) |
|
| InChI
| |
| SMILES
| |
| Backdrop | |
| Chemical formula | Then two |
| Molar mass | 64.066 g mol−1 |
| Appearance | Colorless gas |
| Scent | Pungent; similar to a but-struck match[1] |
| Density | 2.6288 kg m−three [ commendation needed ] |
| Melting betoken | −72 °C; −98 °F; 201 K |
| Humid indicate | −10 °C (14 °F; 263 One thousand) |
| Solubility in water | 94 1000/Fifty[2] forms sulfurous acid |
| Vapor force per unit area | 237.ii kPa |
| Acerbity (pK a) | 1.81 |
| Basicity (pK b) | 12.19 |
| Magnetic susceptibility (χ) | −eighteen.2·10−half-dozen cm3/mol |
| Viscosity | 12.82 μPa·s[three] |
| Structure | |
| Point group | C twov |
| Coordination geometry | Digonal |
| Molecular shape | Dihedral |
| Dipole moment | 1.62 D |
| Thermochemistry | |
| Std molar | 248.223 J K−1 mol−1 |
| Std enthalpy of | −296.81 kJ mol−1 |
| Hazards | |
| GHS labelling: | |
| Pictograms |   |
| Signal word | Danger |
| Hazard statements | H314, H331 [4] |
| NFPA 704 (burn diamond) | three 0 0 |
| Lethal dose or concentration (LD, LC): | |
| LCl (median concentration) | 3000 ppm (mouse, thirty min) 2520 ppm (rat, i 60 minutes)[half dozen] |
| LCLo (everyman published) | 993 ppm (rat, 20 min) 611 ppm (rat, 5 hr) 764 ppm (mouse, 20 min) grand ppm (human, 10 min) 3000 ppm (man, 5 min)[6] |
| NIOSH (Us health exposure limits): | |
| PEL (Permissible) | TWA 5 ppm (13 mg/m3)[five] |
| REL (Recommended) | TWA ii ppm (5 mg/grandthree) ST five ppm (thirteen mg/k3)[five] |
| IDLH (Immediate danger) | 100 ppm[5] |
| Related compounds | |
| Related sulfur oxides | Sulfur monoxide Sulfur trioxide |
| Related compounds | Ozone Selenium dioxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
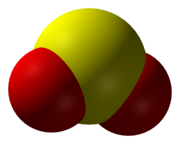
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the compound with the formula SO
2 . Information technology is a toxic gas responsible for the odour of burnt matches. Information technology is released naturally by volcanic activity and is produced every bit a by-product of copper extraction and the called-for of sulfur-bearing fossil fuels. Sulfur dioxide has a pungent smell similar nitric acrid.[ citation needed ]
Structure and bonding [edit]
Thentwo is a bent molecule with C 2v symmetry point group. A valence bond theory approach because just s and p orbitals would describe the bonding in terms of resonance between two resonance structures.

2 resonance structures of sulfur dioxide
The sulfur–oxygen bond has a bail order of 1.5. At that place is support for this unproblematic approach that does not invoke d orbital participation.[seven] In terms of electron-counting formalism, the sulfur atom has an oxidation country of +iv and a formal accuse of +1.
Occurrence [edit]

The blueish auroral glows of Io's upper atmosphere are caused past volcanic sulfur dioxide.
Sulfur dioxide is establish on Earth and exists in very small concentrations and in the atmosphere at about 1 ppm.[8] [nine] [ clarification needed ]
On other planets, sulfur dioxide can be found in various concentrations, the most meaning being the temper of Venus, where it is the third-most arable atmospheric gas at 150 ppm. There, information technology reacts with water to form clouds of sulfuric acid, and is a central component of the planet's global atmospheric sulfur bike and contributes to global warming.[x] It has been implicated as a key agent in the warming of early Mars, with estimates of concentrations in the lower atmosphere as loftier every bit 100 ppm,[eleven] though it only exists in trace amounts. On both Venus and Mars, as on Earth, its primary source is thought to be volcanic. The temper of Io, a natural satellite of Jupiter, is 90% sulfur dioxide[12] and trace amounts are thought to also be in the atmosphere of Jupiter.
Equally an water ice, information technology is idea to exist in abundance on the Galilean moons—as subliming ice or frost on the trailing hemisphere of Io,[thirteen] and in the crust and mantle of Europa, Ganymede, and Callisto, perchance too in liquid form and readily reacting with h2o.[14]
Production [edit]
Sulfur dioxide is primarily produced for sulfuric acid manufacture (see contact procedure). In the United States in 1979, 23.6 million metric tons (26,014,547 Usa short tons) of sulfur dioxide were used in this way, compared with 150 thousand metric tons (165,347 United states of america short tons) used for other purposes. Most sulfur dioxide is produced by the combustion of elemental sulfur. Some sulfur dioxide is also produced by roasting pyrite and other sulfide ores in air.[15]
An experiment showing called-for of sulfur in oxygen. A flow-chamber joined to a gas washing canteen (filled with a solution of methyl orange) is being used. The product is sulfur dioxide (SOii) with some traces of sulfur trioxide (SOthree). The "smoke" that exits the gas washing canteen is, in fact, a sulfuric acid fog generated in the reaction.
Combustion routes [edit]
Sulfur dioxide is the product of the burning of sulfur or of burning materials that contain sulfur:
- South + O2 → And then2, ΔH = −297 kJ/mol
To assist combustion, liquified sulfur (140–150 °C, 284-302 °F) is sprayed through an atomizing nozzle to generate fine drops of sulfur with a large surface area. The reaction is exothermic, and the combustion produces temperatures of grand–1600 °C (1832–2912 °F). The significant corporeality of heat produced is recovered by steam generation that tin can subsequently exist converted to electricity.[15]
The combustion of hydrogen sulfide and organosulfur compounds proceeds similarly. For example:
- 2 H2South + three O2 → ii HiiO + 2 SO2
The roasting of sulfide ores such as pyrite, sphalerite, and cinnabar (mercury sulfide) also releases And then2:[16]
- 4 FeS2 + 11 O2 → 2 Iron2Oiii + eight SOii
- ii ZnS + 3 O2 → ii ZnO + two And so2
- HgS + O2 → Hg + SO2
- 4 FeS + 7Oii → ii Atomic number 262Othree + four SO2
A combination of these reactions is responsible for the largest source of sulfur dioxide, volcanic eruptions. These events can release millions of tons of Soii.
Reduction of college oxides [edit]
Sulfur dioxide can also be a byproduct in the industry of calcium silicate cement; CaSO4 is heated with coke and sand in this procedure:
- 2 CaSO4 + ii SiO2 + C → two CaSiO3 + 2 SO2 + COtwo
Until the 1970s, commercial quantities of sulfuric acid and cement were produced by this procedure in Whitehaven, England. Upon being mixed with shale or marl, and roasted, the sulfate liberated sulfur dioxide gas, used in sulfuric acrid production, the reaction besides produced calcium silicate, a precursor in cement production.[17]
On a laboratory scale, the activeness of hot concentrated sulfuric acid on copper turnings produces sulfur dioxide.
- Cu + 2 H2And soiv → CuSO4 + Then2 + 2 HtwoO
From sulfites [edit]
Sulfites results past the activity of aqueous base on sulfur dioxide:
- And sotwo + 2 NaOH → NaiiAnd so3 + H2O
The reverse reaction occurs upon acidification:
- H+ + HSO3 − → And so2 + H2O
Reactions [edit]
Featuring sulfur in the +4 oxidation state, sulfur dioxide is a reducing agent. Information technology is oxidized by halogens to requite the sulfuryl halides, such as sulfuryl chloride:
- SO2 + Cltwo → So2Cltwo
Sulfur dioxide is the oxidising agent in the Claus procedure, which is conducted on a large scale in oil refineries. Here, sulfur dioxide is reduced by hydrogen sulfide to give elemental sulfur:
- SO2 + two H2S → three S + ii HiiO
The sequential oxidation of sulfur dioxide followed past its hydration is used in the production of sulfuric acrid.
- 2 And so2 + ii H2O + O2 → 2 H2Then4
Laboratory reactions [edit]
Sulfur dioxide is one of the few common acidic yet reducing gases. Information technology turns moist litmus pink (being acidic), and then white (due to its bleaching effect). It may exist identified by bubbling it through a dichromate solution, turning the solution from orange to light-green (Cr3+ (aq)). It can also reduce ferric ions to ferrous.[eighteen]
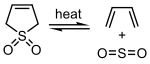
Sulfur dioxide tin can react with certain 1,3-dienes in a cheletropic reaction to form cyclic sulfones. This reaction is exploited on an industrial scale for the synthesis of sulfolane, which is an of import solvent in the petrochemical industry.
Sulfur dioxide can bind to metal ions equally a ligand to class metallic sulfur dioxide complexes, typically where the transition metal is in oxidation land 0 or +1. Many different bonding modes (geometries) are recognized, but in almost cases, the ligand is monodentate, attached to the metal through sulfur, which can exist either planar and pyramidal η1.[19] As a η1-SO2 (Due south-bonded planar) ligand sulfur dioxide functions every bit a Lewis base of operations using the lone pair on South. SOii functions equally a Lewis acids in its ηone-SOtwo (S-bonded pyramidal) bonding style with metals and in its 1:one adducts with Lewis bases such equally dimethylacetamide and trimethyl amine. When bonding to Lewis bases the acid parameters of SO2 are EastwardA = 0.51 and Due eastA = 1.56.
Uses [edit]
The overarching, dominant employ of sulfur dioxide is in the product of sulfuric acid.[fifteen]
Precursor to sulfuric acrid [edit]
Sulfur dioxide is an intermediate in the production of sulfuric acid, being converted to sulfur trioxide, and and so to oleum, which is made into sulfuric acrid. Sulfur dioxide for this purpose is made when sulfur combines with oxygen. The method of converting sulfur dioxide to sulfuric acid is chosen the contact procedure. Several billion kilograms are produced annually for this purpose.
Equally a preservative [edit]
Sulfur dioxide is sometimes used as a preservative for stale apricots, dried figs, and other dried fruits, owing to its antimicrobial properties and ability to prevent oxidation,[twenty] and is called E220[21] when used in this style in Europe. As a preservative, it maintains the colorful appearance of the fruit and prevents rotting. Information technology is also added to sulfured molasses.
Sulfur dioxide was get-go used in winemaking by the Romans, when they discovered that burning sulfur candles inside empty wine vessels keeps them fresh and free from vinegar smell.[22]
Information technology is still an important compound in winemaking, and is measured in parts per million (ppm) in wine. It is present even in and then-called unsulfurated vino at concentrations of up to 10 mg/50.[23] It serves as an antibiotic and antioxidant, protecting wine from spoilage by leaner and oxidation - a phenomenon that leads to the browning of the wine and a loss of cultivar specific flavors.[24] [25] Its antimicrobial action too helps minimize volatile acidity. Wines containing sulfur dioxide are typically labeled with "containing sulfites".
Sulfur dioxide exists in wine in free and spring forms, and the combinations are referred to as total And then2. Bounden, for case to the carbonyl group of acetaldehyde, varies with the wine in question. The costless class exists in equilibrium between molecular So2 (as a dissolved gas) and bisulfite ion, which is in turn in equilibrium with sulfite ion. These equilibria depend on the pH of the wine. Lower pH shifts the equilibrium towards molecular (gaseous) SO2, which is the active form, while at higher pH more SO2 is found in the inactive sulfite and bisulfite forms. The molecular And then2 is agile equally an antimicrobial and antioxidant, and this is besides the form which may be perceived as a pungent odor at high levels. Wines with total And sotwo concentrations below ten ppm do non require "contains sulfites" on the label by The states and EU laws. The upper limit of total SO2 allowed in vino in the US is 350 ppm; in the European union information technology is 160 ppm for scarlet wines and 210 ppm for white and rosé wines. In low concentrations, So2 is mostly undetectable in wine, simply at free And then2 concentrations over 50 ppm, SO2 becomes evident in the smell and taste of vino.[ citation needed ]
SO2 is also a very important compound in winery sanitation. Wineries and equipment must be kept make clean, and because bleach cannot be used in a winery due to the risk of cork taint,[26] a mixture of And thentwo, h2o, and citric acid is commonly used to clean and sanitize equipment. Ozone (Oiii) is now used extensively for sanitizing in wineries due to its efficacy, and because it does not affect the wine or most equipment.[27]
Every bit a reducing agent [edit]
Sulfur dioxide is also a good reductant. In the presence of water, sulfur dioxide is able to decolorize substances. Specifically, it is a useful reducing bleach for papers and delicate materials such as wearing apparel. This bleaching effect commonly does not concluding very long. Oxygen in the atmosphere reoxidizes the reduced dyes, restoring the color. In municipal wastewater treatment, sulfur dioxide is used to treat chlorinated wastewater prior to release. Sulfur dioxide reduces free and combined chlorine to chloride.[28]
Sulfur dioxide is fairly soluble in water, and past both IR and Raman spectroscopy; the hypothetical sulfurous acid, HtwoTheniii, is not present to whatsoever extent. However, such solutions do show spectra of the hydrogen sulfite ion, HSOiii −, by reaction with water, and it is in fact the actual reducing agent present:
- SO2 + HtwoO ⇌ HSO3 − + H+
Equally a fumigant [edit]
In the beginning of the 20th century, sulfur dioxide was used in Buenos Aires as a fumigant to kill rats that carried the Yersinia pestis bacterium, which causes bubonic plague. The awarding was successful, and the application of this method was extended to other areas in Due south America. In Buenos Aires, where these apparatuses were known as Sulfurozador, but later also in Rio de Janeiro, New Orleans and San Francisco, the sulfur dioxide treatment machines were brought into the streets to enable extensive disinfection campaigns, with effective results.[29]
Biochemical and biomedical roles [edit]
Sulfur dioxide or its conjugate base bisulfite is produced biologically equally an intermediate in both sulfate-reducing organisms and in sulfur-oxidizing bacteria, as well. The role of sulfur dioxide in mammalian biology is not yet well understood.[30] Sulfur dioxide blocks nerve signals from the pulmonary stretch receptors and abolishes the Hering–Breuer inflation reflex.
It is considered that endogenous sulfur dioxide plays a significant physiological function in regulating cardiac and blood vessel role, and aberrant or deficient sulfur dioxide metabolism can contribute to several different cardiovascular diseases, such as arterial hypertension, atherosclerosis, pulmonary arterial hypertension, and stenocardia.[31]
Information technology was shown that in children with pulmonary arterial hypertension due to congenital center diseases the level of homocysteine is higher and the level of endogenous sulfur dioxide is lower than in normal command children. Moreover, these biochemical parameters strongly correlated to the severity of pulmonary arterial hypertension. Authors considered homocysteine to be one of useful biochemical markers of illness severity and sulfur dioxide metabolism to be 1 of potential therapeutic targets in those patients.[32]
Endogenous sulfur dioxide likewise has been shown to lower the proliferation rate of endothelial smooth musculus cells in blood vessels, via lowering the MAPK activity and activating adenylyl cyclase and poly peptide kinase A.[33] Smooth muscle cell proliferation is one of of import mechanisms of hypertensive remodeling of claret vessels and their stenosis, so information technology is an important pathogenetic mechanism in arterial hypertension and atherosclerosis.
Endogenous sulfur dioxide in low concentrations causes endothelium-dependent vasodilation. In higher concentrations it causes endothelium-contained vasodilation and has a negative inotropic effect on cardiac output function, thus effectively lowering blood pressure and myocardial oxygen consumption. The vasodilating and bronchodilating effects of sulfur dioxide are mediated via ATP-dependent calcium channels and 50-type ("dihydropyridine") calcium channels. Endogenous sulfur dioxide is besides a potent antiinflammatory, antioxidant and cytoprotective agent. It lowers claret pressure level and slows hypertensive remodeling of blood vessels, peculiarly thickening of their intima. Information technology also regulates lipid metabolism.[34]
Endogenous sulfur dioxide also diminishes myocardial impairment, caused by isoproterenol adrenergic hyperstimulation, and strengthens the myocardial antioxidant defence force reserve.[35]
Every bit a reagent and solvent in the laboratory [edit]
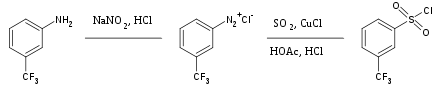
Sulfur dioxide is a versatile inert solvent widely used for dissolving highly oxidizing salts. Information technology is also used occasionally as a source of the sulfonyl group in organic synthesis. Handling of aryl diazonium salts with sulfur dioxide and cuprous chloride yields the corresponding aryl sulfonyl chloride, for instance:[36]
Every bit a upshot of its very low Lewis basicity, it is often used as a low-temperature solvent/diluent for superacids like magic acid (FSO3H/SbF5), assuasive for highly reactive species like tert-butyl cation to be observed spectroscopically at low temperature (though tertiary carbocations practise react with SO2 above almost –30 °C, and fifty-fifty less reactive solvents like And so2ClF must be used at these college temperatures).[37]
Aspirational applications [edit]
Every bit a refrigerant [edit]
Existence easily condensed and possessing a high estrus of evaporation, sulfur dioxide is a candidate material for refrigerants. Prior to the development of chlorofluorocarbons, sulfur dioxide was used as a refrigerant in home refrigerators.
Climate engineering [edit]
Injections of sulfur dioxide in the stratosphere has been proposed in climate technology. The cooling result would be like to what has been observed afterwards the large explosive 1991 eruption of Mount Pinatubo. However this class of geoengineering would accept uncertain regional consequences on rainfall patterns, for case in monsoon regions.[38]
Equally an air pollutant [edit]

A collection of estimates of past and future anthropogenic global sulfur dioxide emissions. The Cofala et al. estimates are for sensitivity studies on SO2 emission policies, CLE: Current Legislation, MFR: Maximum Feasible Reductions. RCPs (Representative Concentration Pathways) are used in CMIP5 simulations for latest (2013–2014) IPCC fifth assessment report.
Sulfur dioxide is a noticeable component in the atmosphere, particularly following volcanic eruptions.[39] According to the United States Environmental Protection Agency (EPA),[40] the amount of sulfur dioxide released in the U.S. per year was:
| Yr | And so2 |
|---|---|
| 1970 | 31,161,000 short tons (28.3 Mt) |
| 1980 | 25,905,000 brusk tons (23.5 Mt) |
| 1990 | 23,678,000 short tons (21.5 Mt) |
| 1996 | eighteen,859,000 short tons (17.1 Mt) |
| 1997 | xix,363,000 short tons (17.six Mt) |
| 1998 | xix,491,000 brusque tons (17.7 Mt) |
| 1999 | eighteen,867,000 short tons (17.one Mt) |
Sulfur dioxide is a major air pollutant and has significant impacts upon homo wellness.[41] In addition, the concentration of sulfur dioxide in the atmosphere can influence the habitat suitability for plant communities, as well as animal life.[42] Sulfur dioxide emissions are a precursor to acid rain and atmospheric particulates. Due largely to the US EPA's Acid Rain Program, the U.S. has had a 33% decrease in emissions between 1983 and 2002. This comeback resulted in office from flue-gas desulfurization, a technology that enables SOii to be chemically bound in power plants burning sulfur-containing coal or oil. In particular, calcium oxide (lime) reacts with sulfur dioxide to grade calcium sulfite:
- CaO + So2 → CaSO3
Aerobic oxidation of the CaSO3 gives CaSO4, anhydrite. Most gypsum sold in Europe comes from flue-gas desulfurization.
To control sulfur emissions, dozens of methods with relatively loftier efficiencies take been adult for plumbing fixtures of coal-fired power plants.[43]
Sulfur tin can be removed from coal during burning by using limestone as a bed material in fluidized bed combustion.[44]
Sulfur tin also exist removed from fuels earlier burning, preventing germination of SO2 when the fuel is burnt. The Claus process is used in refineries to produce sulfur as a byproduct. The Stretford procedure has also been used to remove sulfur from fuel. Redox processes using atomic number 26 oxides tin can besides exist used, for example, Lo-Cat[45] or Sulferox.[46]
An assay plant that 18 coal-fired power stations in the western Balkans emitted two-and-half times more sulphur dioxide than all 221 coal plants in the Eu combined.[47]
Fuel additives such as calcium additives and magnesium carboxylate may be used in marine engines to lower the emission of sulfur dioxide gases into the temper.[48]
Equally of 2006, People's republic of china was the world's largest sulfur dioxide polluter, with 2005 emissions estimated to be 25,490,000 short tons (23.i Mt). This amount represents a 27% increment since 2000, and is roughly comparable with U.S. emissions in 1980.[49]
-

A sulfur dioxide plumage from Halemaʻumaʻu, which glows at nighttime
-

Sulfur dioxide in the globe on April 15, 2017. Note that sulfur dioxide moves through the temper with prevailing winds and thus local sulfur dioxide distributions vary twenty-four hours to mean solar day with weather patterns and seasonality.
Safety [edit]

Inhalation [edit]
Incidental exposure to sulfur dioxide is routine, e.g. the smoke from matches, coal, and sulfur-containing fuels.
Sulfur dioxide is mildly toxic and can be hazardous in loftier concentrations.[50] Long-term exposure to low concentrations is also problematic. A 2011 systematic review ended that exposure to sulfur dioxide is associated with preterm birth.[51]
U.South. regulations [edit]
In 2008, the American Conference of Governmental Industrial Hygienists reduced the short-term exposure limit to 0.25 parts per million (ppm). In the Usa, the OSHA set the PEL at 5 ppm (13 mg/g3) time-weighted average. Besides in the The states, NIOSH ready the IDLH at 100 ppm.[52] In 2010, the EPA "revised the chief And so2 NAAQS past establishing a new one-hour standard at a level of 75 parts per billion (ppb). EPA revoked the two existing primary standards because they would not provide additional public health protection given a one-60 minutes standard at 75 ppb."[41]
Ingestion [edit]
In the United States, the Center for Science in the Public Involvement lists the two nutrient preservatives, sulfur dioxide and sodium bisulfite, as being safety for human consumption except for sure asthmatic individuals who may be sensitive to them, especially in big amounts.[53] Symptoms of sensitivity to sulfiting agents, including sulfur dioxide, manifest as potentially life-threatening trouble breathing within minutes of ingestion.[54] Sulphites may besides cause symptoms in non-asthmatic individuals, namely dermatitis, urticaria, flushing, hypotension, abdominal pain and diarrhea, and even life-threatening anaphylaxis.[55]
Encounter also [edit]
- Bunker fuel
- National Ambient Air Quality Standards
- Sulfur trioxide
- Sulfur–iodine bike
References [edit]
- ^ Sulfur dioxide Archived 2019-12-30 at the Wayback Motorcar, U.S. National Library of Medicine
- ^ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN0-8493-0487-3.
- ^ Miller, J.W. Jr.; Shah, P.Northward.; Yaws, C.L. (1976). "Correlation constants for chemic compounds". Chemical Engineering. 83 (25): 153–180. ISSN 0009-2460.
- ^ "C&L Inventory".
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0575". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Sulfur dioxide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Rubber and Health (NIOSH).
- ^ Cunningham, Terence P.; Cooper, David L.; Gerratt, Joseph; Karadakov, Peter B. & Raimondi, Mario (1997). "Chemic bonding in oxofluorides of hypercoordinatesulfur". Journal of the Chemic Society, Faraday Transactions. 93 (13): 2247–2254. doi:10.1039/A700708F.
- ^ Owen, Lewis A.; Pickering, Kevin T (1997). An Introduction to Global Environmental Problems. Taylor copper extraction& Francis. pp. 33–. ISBN978-0-203-97400-1.
- ^ Taylor, J.A.; Simpson, R.W.; Jakeman, A.J. (1987). "A hybrid model for predicting the distribution of sulphur dioxide concentrations observed near elevated betoken sources". Ecological Modelling. 36 (3–4): 269–296. doi:x.1016/0304-3800(87)90071-8. ISSN 0304-3800.
- ^ Marcq, Emmanuel; Bertaux, Jean-Loup; Montmessin, Franck; Belyaev, Denis (2012). "Variations of sulphur dioxide at the cloud height of Venus's dynamic temper". Nature Geoscience. half dozen: 25–28. Bibcode:2013NatGe...half-dozen...25M. doi:x.1038/ngeo1650. ISSN 1752-0894. S2CID 59323909.
- ^ Halevy, I.; Zuber, M. T.; Schrag, D. P. (2007). "A Sulfur Dioxide Climate Feedback on Early Mars". Science. 318 (5858): 1903–1907. Bibcode:2007Sci...318.1903H. doi:10.1126/science.1147039. ISSN 0036-8075. PMID 18096802. S2CID 7246517.
- ^ Lellouch, East.; et al. (2007). "Io's atmosphere". In Lopes, R. Yard. C.; Spencer, J. R. (eds.). Io after Galileo. Springer-Praxis. pp. 231–264. ISBN978-iii-540-34681-iv.
- ^ Cruikshank, D. P.; Howell, R. R.; Geballe, T. R.; Fanale, F. P. (1985). "Sulfur Dioxide Ice on IO". ICES in the Solar System. pp. 805–815. doi:10.1007/978-94-009-5418-2_55. ISBN978-94-010-8891-6.
- ^ Europa's Hidden Water ice Chemistry – NASA Jet Propulsion Laboratory. Jpl.nasa.gov (2010-ten-04). Retrieved on 2013-09-24.
- ^ a b c Müller, Hermann. "Sulfur Dioxide". Ullmann'southward Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_569.
- ^ Shriver, Atkins. Inorganic Chemistry, Fifth Edition. W. H. Freeman and Company; New York, 2010; p. 414.
- ^ WHITEHAVEN COAST ARCHAEOLOGICAL SURVEY. lakestay.co.uk (2007)
- ^ "Data archivée dans le Web" (PDF).
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemical science of the Elements (second ed.). Butterworth-Heinemann. ISBN978-0-08-037941-8.
- ^ Zamboni, Cibele B.; Medeiros, Ilca M. M. A.; de Medeiros, José A. G. (October 2011). Analysis of Sulfur in Dried Fruits Using NAA (PDF). 2011 International Nuclear Atlantic Conference - INAC 2011. ISBN978-85-99141-03-8.
- ^ Electric current European union approved additives and their E Numbers, The Food Standards Agency website.
- ^ "Practical Winery & vineyard Journal Jan/Feb 2009". www.practicalwinery.com. one Feb 2009. Archived from the original on 2013-09-28.
- ^ Sulphites in wine, MoreThanOrganic.com.
- ^ Jackson, R.S. (2008) Wine science: principles and applications, Amsterdam; Boston: Elsevier/Bookish Printing
- ^ Guerrero, Raúl F; Cantos-Villar, Emma (2015). "Demonstrating the efficiency of sulphur dioxide replacements in wine: A parameter review". Trends in Food Science & Technology. 42: 27–43. doi:10.1016/j.tifs.2014.11.004.
- ^ Chlorine Employ in the Winery. Purdue University
- ^ Use of ozone for winery and environmental sanitation, Practical Winery & Vineyard Journal.
- ^ Tchobanoglous, George (1979). Wastewater Engineering science (3rd ed.). New York: McGraw Hill. ISBN0-07-041677-Ten.
- ^ Engelmann, Lukas (July 2018). "Fumigating the Hygienic Model Metropolis: Bubonic Plague and the Sulfurozador in Early-Twentieth-Century Buenos Aires". Medical History. 62 (3): 360–382. doi:10.1017/mdh.2018.37. PMC6113751. PMID 29886876.
- ^ Liu, D.; Jin, H.; Tang, C.; Du, J. (2010). "Sulfur Dioxide: a Novel Gaseous Signal in the Regulation of Cardiovascular Functions". Mini-Reviews in Medicinal Chemistry. 10 (11): 1039–1045. doi:ten.2174/1389557511009011039. PMID 20540708.
- ^ Tian, Hong (5 November 2014). "Advances in the report on endogenous sulfur dioxide in the cardiovascular system". Chinese Medical Periodical. 127 (21): 3803–3807. doi:10.3760/cma.j.issn.0366-6999.20133031 (inactive 28 Feb 2022). PMID 25382339.
{{cite periodical}}: CS1 maint: DOI inactive as of February 2022 (link) - ^ Yang R, Yang Y, Dong Ten, Wu X, Wei Y (Aug 2014). "Correlation between endogenous sulfur dioxide and homocysteine in children with pulmonary arterial hypertension associated with congenital heart disease". Zhonghua Er Ke Za Zhi (in Chinese). 52 (8): 625–629. PMID 25224243.
- ^ Liu D, Huang Y, Bu D, Liu AD, Holmberg Fifty, Jia Y, Tang C, Du J, Jin H (May 2014). "Sulfur dioxide inhibits vascular smooth musculus cell proliferation via suppressing the Erk/MAP kinase pathway mediated by cAMP/PKA signaling". Cell Death Dis. v (5): e1251. doi:10.1038/cddis.2014.229. PMC4047873. PMID 24853429.
- ^ Wang XB, Jin HF, Tang CS, Du JB (sixteen Nov 2011). "The biological issue of endogenous sulfur dioxide in the cardiovascular system". Eur J Pharmacol. 670 (1): 1–six. doi:10.1016/j.ejphar.2011.08.031. PMID 21925165.
- ^ Liang Y, Liu D, Ochs T, Tang C, Chen Due south, Zhang S, Geng B, Jin H, Du J (January 2011). "Endogenous sulfur dioxide protects against isoproterenol-induced myocardial injury and increases myocardial antioxidant capacity in rats". Lab. Invest. 91 (ane): 12–23. doi:10.1038/labinvest.2010.156. PMID 20733562.
- ^ Hoffman, R. V. (1990). "m-Trifluoromethylbenzenesulfonyl Chloride". Organic Syntheses. ; Commonage Book, vol. seven, p. 508
- ^ Olah, George A.; Lukas, Joachim. (1967-08-01). "Stable carbonium ions. XLVII. Alkylcarbonium ion formation from alkanes via hydride (alkide) ion brainchild in fluorosulfonic acid-antimony pentafluoride-sulfuryl chlorofluoride solution". Journal of the American Chemical Gild. 89 (18): 4739–4744. doi:10.1021/ja00994a030. ISSN 0002-7863.
- ^ Clarke Fifty., K. Jiang, Thousand. Akimoto, Thousand. Babiker, G. Blanford, K. Fisher-Vanden, J.-C. Hourcade, Five. Krey, E. Kriegler, A. Löschel, D. McCollum, S. Paltsev, Due south. Rose, P. R. Shukla, M. Tavoni, B. C. C. van der Zwaan, and D.P. van Vuuren, 2014: Assessing Transformation Pathways. In: Climate change 2014: Mitigation of Climate Modify. Contribution of Working Grouping Iii to the Fifth Assessment Study of the Intergovernmental Panel on Climate change [Edenhofer, O., R. Pichs-Madruga, Y. Sokona, E. Farahani, Southward. Kadner, K. Seyboth, A. Adler, I. Baum, S. Brunner, P. Eickemeier, B. Kriemann, J. Savolainen, Southward. Schlömer, C. von Stechow, T. Zwickel and J.C. Minx (eds.)]. Cambridge University Press, Cambridge, Uk and New York, NY, USA.
- ^ Volcanic Gases and Their Furnishings. Volcanoes.usgs.gov. Retrieved on 2011-x-31.
- ^ National Trends in Sulfur Dioxide Levels, United states of america Environmental Protection Agency.
- ^ a b Sulfur Dioxide (SO2) Pollution. Us Environmental Protection Agency
- ^ Hogan, C. Michael (2010). "Abiotic factor" in Encyclopedia of Earth. Emily Monosson and C. Cleveland (eds.). National Council for Science and the Environs. Washington DC
- ^ Lin, Cheng-Kuan; Lin, Ro-Ting; Chen, Pi-Cheng; Wang, Pu; De Marcellis-Warin, Nathalie; Zigler, Corwin; Christiani, David C. (2018-02-08). "A Global Perspective on Sulfur Oxide Controls in Coal-Fired Power Plants and Cardiovascular Affliction". Scientific Reports. viii (1): 2611. Bibcode:2018NatSR...8.2611L. doi:x.1038/s41598-018-20404-2. ISSN 2045-2322. PMC5805744. PMID 29422539.
- ^ Lindeburg, Michael R. (2006). Mechanical Technology Reference Manual for the PE Exam. Belmont, C.A.: Professional person Publications, Inc. pp. 27–3. ISBN978-i-59126-049-iii.
- ^ FAQ's Well-nigh Sulfur Removal and Recovery using the LO-True cat® Hydrogen Sulfide Removal System. gtp-merichem.com
- ^ Process screening analysis of alternative gas treating and sulfur removal for gasification. (December 2002) Report by SFA Pacific, Inc. prepared for U.S. Section of Free energy (PDF) . Retrieved on 2011-10-31.
- ^ Carrington, Damian (2021-09-06). "More than global assist goes to fossil fuel projects than tackling dingy air – study". The Guardian . Retrieved 2021-09-07 .
{{cite spider web}}: CS1 maint: url-status (link) - ^ May, Walter R. Marine Emissions Abatement Archived 2015-04-02 at the Wayback Machine. SFA International, Inc., p. half dozen.
- ^ Mainland china has its worst spell of acid rain, United Press International (2006-09-22).
- ^ Sulfur Dioxide Nuts U.South. Ecology Protection Agency
- ^ Shah PS, Balkhair T, Knowledge Synthesis Group on Determinants of Preterm/LBW Births (2011). "Air pollution and birth outcomes: a systematic review". Environ Int. 37 (two): 498–516. doi:10.1016/j.envint.2010.ten.009. PMID 21112090.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "NIOSH Pocket Guide to Chemical Hazards".
- ^ "Center for Science in the Public Interest – Chemical Cuisine". Retrieved March 17, 2010.
- ^ "California Department of Public Wellness: Food and Drug Co-operative: Sulfites" (PDF). Archived from the original (PDF) on July 23, 2012. Retrieved September 27, 2013.
- ^ Vally H, Misso NL (2012). "Adverse reactions to the sulphite additives". Gastroenterol Hepatol Bed Bench. five (1): 16–23. PMC4017440. PMID 24834193.
External links [edit]
- Global map of sulfur dioxide distribution
- Usa Ecology Protection Agency Sulfur Dioxide page
- International Chemic Prophylactic Card 0074
- IARC Monographs. "Sulfur Dioxide and some Sulfites, Bisulfites and Metabisulfites". vol. 54. 1992. p. 131.
- NIOSH Pocket Guide to Chemical Hazards
- CDC – Sulfure Dioxide – NIOSH Workplace Safety and Health Topic
- Sulfur Dioxide, Molecule of the Month
shepherdthallactle.blogspot.com
Source: https://en.wikipedia.org/wiki/Sulfur_dioxide

0 Response to "Market Oriented Policies Agains Sulfur Dioxide"
Postar um comentário